Dispersing Agent
December 06, 2022 · Dhanya
Dispersants, also called dispersing agents, are chemical agents used to break up oil into smaller droplets in the water column. Dispersants can be applied on surface oil or below the surface, closer to an uncontrolled release of crude oil from a well blowout source.
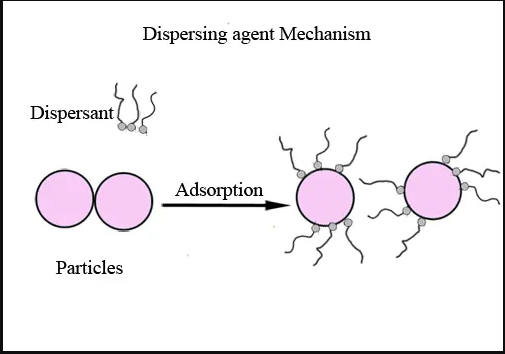
Dispersing agents are specialized wetting agents that wet out surfaces of particles during the dispersion process, and subsequently stabilize the dispersion such that the particles do not re-agglomerate. Dispersing agents prevent re-agglomeration by either electrostatic repulsion or by steric hindrance. Dispersing agents consist of a polar head that "attaches" to the particle to be dispersed, and a polymer chain that is compatible in the media in which the particle is being dispersed. Check out Rubber chemical dispersion manufacturer in kerala
What is the purpose of dispersant?
Dispersants are chemicals that are sprayed on a surface oil slick to break down the oil into smaller droplets that more readily mix with the water. Dispersants do not reduce the amount of oil entering the environment, but push the effects of the spill underwater.
Our product ‘Damol’ is an anionic dispersing additive, tailor-made according to the need of making Chemical dispersions especially for the Rubber and Textile sector. The compound is designed with basic knowledge about the raw materials and corresponding production process of dispersions. The product characteristics are as follows:-
A-Specifications
(1) Physical form Brown coloured Spray dried powder
(2) Chemical nature Anionic in nature
(3) Solubility Soluble in water
(4) PH (1%Solution) 7±1
(5) Active nature About 82%
(6) Moisture content 5% max
Action of dispersing agent in water based paint/ coating
One of the most difficult processes in manufacturing pigmented systems is the process of dispersing pigments and trying to prevent from flocculation occurring. Flocculation is the recombination of dispersed pigments that are not stabilized in dispersions. In water-based systems, the problems are exacerbated due to the high surface tension of water which makes the wetting of low surface energy pigments difficult, especially for organic pigments. Inorganic pigments like titanium dioxide are relatively easy to disperse in water-based systems and dispersants with anionic properties are usually the products of choice.
However, as mentioned organic pigments have low surface energies but also have high surface areas and more complex dispersants are required.
There are generally 3 steps in the process of dispersing pigments:
- Wetting
- Dispersion (grinding)
- Stabilization
Before we discuss the 3 important steps of dispersion of pigments, we need some definitions of pigment particles.
Primary particles are single (or double) crystals and are the smallest particles obtained during pigment synthesis and their simple shapes can vary between cubic, rhomboidal etc.
Aggregates are rigid clusters of primary particles joined by crystal faces. Due to the high adhesion forces, dispersion does not cause aggregates to separate into primary particles.
Agglomerates are clusters of primary particles or aggregates that are loosely joined by crystal edges due to the action of van der Waals or Coulombic forces. These can be broken down, so the entire process of pigment dispersion is to divide agglomerates into primary particles and aggregates. In practice, large agglomerates usually end up being divided into smaller agglomerates.
1) Wetting is the first step of the dispersion process in which air and moisture are displaced from the pigment surface by the dispersing agent. This is done through preferential adsorption. The efficiency of wetting depends primarily on the surface tension properties between the pigment and the vehicle, as well as the viscosity of the resultant mix. The air must be fully removed and the pigment surrounded by the liquid medium. The action of wetting can cause surface tension to be lowered.
This means that a liquid with low surface tension will wet the pigment surface better than a high surface tension liquid so that a dispersant that acts as a wetting agent in this first step will lower the surface tension.
2) Dispersion (grinding) stage is where the primary purpose is to break down pigment aggregates and agglomerates to their optimum particulate size by milling machines or in the case of inorganic pigments the use of a high-speed mixer using a saw tooth blade. The dispersant additive speeds up the wetting of the newly created surfaces and minimizes the pigment – pigment interaction to produce a lower viscosity dispersion. During this process, the pigment particles are stabilized against flocculation and without this stabilization, the primary particles would re-agglomerate releasing the energy that was introduced into the dispersion by the grinding process.
Dispersants reduce the surface tension, and from the above equation, the work needed to disperse the pigment is less. This means that the grinding time is reduced.
3) Stabilization is the most critical stage of the process and the dispersant needs to have good wetting and dispersing properties to be able, over a period of time, to keep the particles separated. If this does not happen then the particles can re-form to form flocculates. Flocculation is due to the London - Van der Waal attractive forces occurring between the particles. These forces are only effective over very small distances. In this instance, Brownian molecular motion causes particles to collide many times and creates flocculation.
The dispersant which anchors onto the pigment surface helps to repel the attractive forces between the pigment particles and stops the formation of agglomerates. This requires the dispersant to have groups which interact strongly with the pigment surface by a variety of bonding mechanisms. This stabilization of the pigment ensures that complete wetting and separation of the particles has been reached, and that the pigment particles are homogeneously distributed in the medium. If the dispersion has not been stabilized, flocculation may occur as a result of clumping together of the pigment particles. Flocculation is generally a reversible process. Flocculates typically break down when shear is applied and will form again when the shear is removed. If the pigment dispersion is not stabilized by the resin component in the dispersion, then the use of surfactants or dispersants can be considered.
Quick Enquiry
To know more about Associated Chemicals feel free to send a message
 Our Sister Concerns
Our Sister Concerns 


Usefull Links
Get In Touch
Assochem Chambers, Bypass, Edapally,
Kochi-682024, Kerala, India.
Phones : +91 9495999349, +91 9388610189, +91 484 2339190, +91 484 2348028
E-mail : nsn@assochem.in, marketing@assochem.in, mail@assochem.in
Support